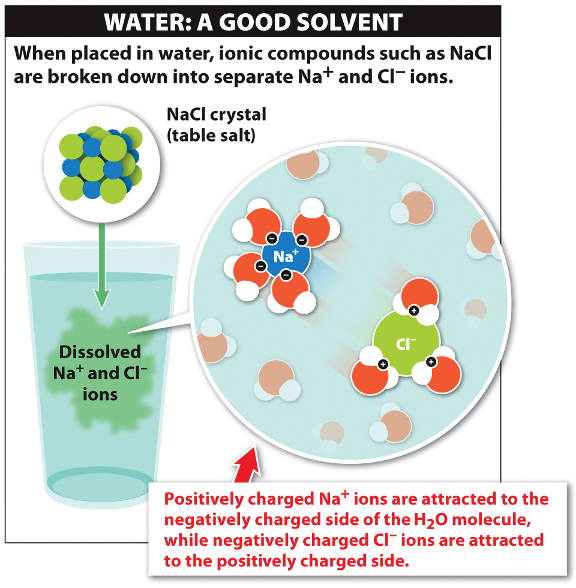
 The partial charges on water molecules are attracted to the charged
sodium
and
chloride
ions of salt (NaCl).
The partial charges on water molecules are attracted to the charged
sodium
and
chloride
ions of salt (NaCl).
The water molecules separate the ions from the crystal and dissolve the salt to make a solution.
Substances such as NaCl dissolved in liquid are called solutes.
Water is a good solvent for ionic or polar substances, which are said to be "hydrophilic" (water-loving) and form a solution in water.