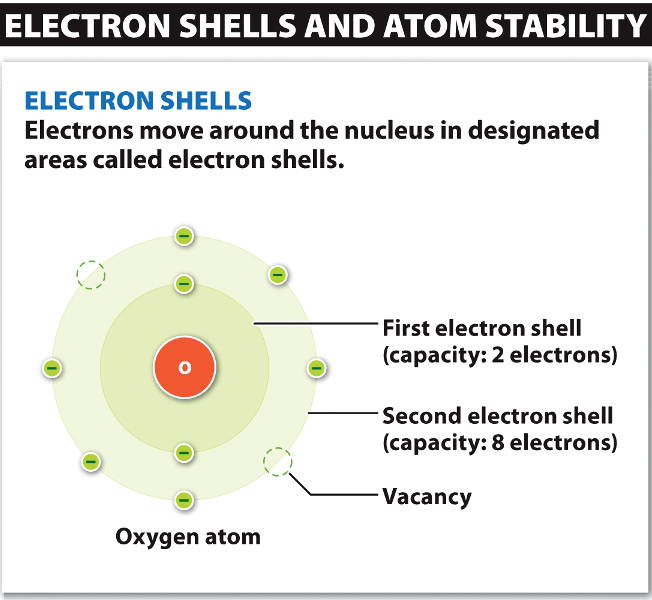
 Electrons
circulate around the nucleus with different energy levels called electron shells.
Electrons
circulate around the nucleus with different energy levels called electron shells.
Lower electron shells possess lower energy, and electrons tend to occupy lower shells first, since they are most stable there.
The 1st shell can hold up to 2 electrons.
The 2nd and 3rd shells can hold up to 8 electrons.