
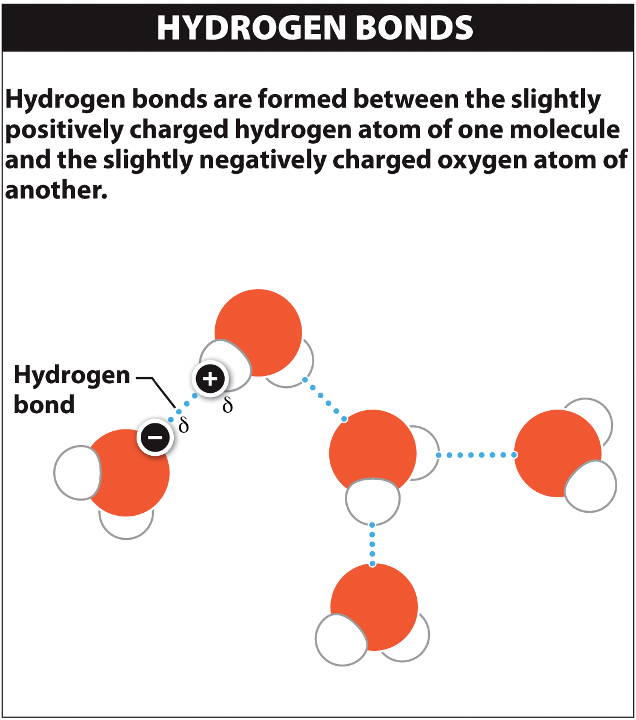
 Hydrogen bond
Hydrogen bond
The partial (δ) positive charges of the hydrogen atoms of one water molecule (H2O) are attracted to the partial (δ) negative charge of the oxygen atom of another molecule.
These weak attractions (dotted lines in the diagram) between polar molecules are hydrogen bonds.
Note that the strong bonds between hydrogen and oxygen atoms within water molecules are covalent bonds.